Publications
Below are the latest publications from the Tuttle Lab.
To stay up to date on the latest publications and events in the Tuttle Lab – follow us on twitter…
-

141. Iridium-Catalysed C(sp3)−H Activation and Hydrogen Isotope Exchange via Nitrogen-Based Carbonyl Directing Groups
Adv. Synth. Catal. 2024 doi.org/10.1002/adsc.202400156
Knight, N. M. L,; Thompson, J. D. F.; Parkinson, J. A.; Lindsay, D. M.; Tuttle, T.; Kerr, W. J.
Adv. Synth. Catal. 2024 doi.org/10.1002/adsc.202400156
-
140. A multifunctional drug delivery system based on switchable peptide-stabilized emulsions
CHEM, 2024,
Boas, D.; van Teijlingen, A.; Shpilt, Z.; Shalev, D. E.; Tshuva, E. Y.; Tuttle, T.; Reches, M.
CHEM 2024 DOI: https://doi.org/10.1016/j.chempr.2024.02.003
-
139. Mathematical and computational modeling of fats and triacylglycerides
Compr. Rev. Food Sci. Food Saf. 2024, 23, e13316.
Cordina, R. J.; Smith, B.; Tuttle, T.
Compr. Rev. Food Sci. Food Saf. 2024 23 e13316
-
138. Martinoid: the peptoid martini force field
Phys. Chem. Chem. Phys. 2024, 26, 4939.
Swanson, H. W. A.; Teijlingen, A. V.; Lau, K. H. A.; Tuttle, T.
Phys. Chem. Chem. Phys. 2024 26 4939.
-
137. Minimal peptoid dynamics inform self-assembly propensity
Swanson, H. W. A.; Lau, K. H. A.; Tuttle, T.
J. Phys. Chem. B 2023 127 10601
-
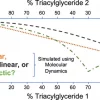
136. Predicting lipid eutectics using coarse-grained molecular dynamics
Cordina, R. J.; Smith, B.; Tuttle, T.
J. Phys. Chem. B 2023 127 10236
-
135. [3+ 2]‐Cycloaddition Reactions of gem‐Difluorocyclopropenes with Azomethine Ylides–Access to Novel Fluorinated Scaffolds
Chem. Eur. J. 2023, e202301861, 10.1002/chem.202301861
Donnelly, K.; Singh, A.; Tuttle, T.; Baumann, M.
Chem. Eur. J. 2023 e202301861
-
134. Alkali Metal Dihydropyridines in Transfer Hydrogenation Catalysis of Imines: Amide Basicity versus Hydride Surrogacy
Angew. Chem. Int. Ed., 2023, e202304966, 10.1002/anie.202304966
Macdonald, P. A.; Banerjee, S,; Kennedy, A. R.; van Teijlingen, A.; Robertson, S. D.; Tuttle, T.; Mulvey, R. E.
Angew. Chem. Int. Ed. 2023 e202304966
-

133. Triacylglyceride Melting Point Determination Using Coarse-Grained Molecular Dynamics
J. Comput. Chem., 2023, 44, 1795.
Cordina, R. J.; Smith, B.; Tuttle, T.
J. Comput. Chem. 2023 44 1795.
-
132. Peptoid Self-Assembly: From Minimal Sequences to Functional Nanoassemblies and Biomedical Applications
Supramolecular Nanotechnology, 2023, 10.1002/9783527834044.ch36
Swanson, H. A. W.; El Yaagoubi, M.; Jawed, A.; Saxena, V.; Merrilees, M.; Tuttle, T.; Pandey, L. M.; Hamley, I. W.; Lau, K. H. A.
Supramolecular Nanotechnology 2023
-
131. Computational Modeling of 4d and 5d Transition Metal Catalysts
Chemistry, Molecular Sciences and Chemical Engineering, 2023, 10.1016/B978-0-12-821978-2.00065-9
Urquhart, R. J.; Tuttle, T.
Chemistry, Molecular Sciences and Chemical Engineering 2023
-

130. Short Peptide Self-Assembly in the Martini Coarse-Grain Force Field Family
Acc. Chem. Res., 2023, 56, 644−654
van Teijlingen, A.; Smith, M. C.; Tuttle, T.
Acc. Chem. Res. 2023 56 644-654
-
129. Integrating Computation, Experiment, and Machine Learning in the Design of Peptide-Based Supramolecular Materials and Systems
Angew. Chem. Int. Ed., 2023, 62, e202218067
Ramakrishnan, M.; van Teijlingen, A.; Tuttle, T.
Angew. Chem. Int. Ed. 2023 62 e202218067
-
128. COGITO: A Coarse-Grained Force Field for the Simulation of Macroscopic Properties of Triacylglycerides
Acc. Chem. Res. 2023, 19, 4, 1333-1341
Cordina, R. J.; Smith, B.; Tuttle, T.
J. Chem. Theory Comput. 2023 19 1333-1341
-

127. Rapid Automated Quantification of Triacylglyceride Crystallinity in Molecular Dynamics Simulations
J. Chem. Inf. Model. 2022, 62, 22, 5601–5606
Cordina, R. J.; Smith, B.; Tuttle, T.
J. Chem. Inf. Model. 2022 62 5601-5606
-
126. Catalytic hydrophosphination of alkynes using structurally diverse sodium diphenylphosphide donor complexes
Cell Reports Physical Science., 2022, 100942
Whitelaw, M.; Banerjee, S.; Kennedy, A.; van Teijlingen, A.; Tuttle, T.; Mulvey, R.
Cell Reports Physical Chemistry 2022 100942
-
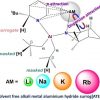
125. Hydrocarbon Soluble Alkali-Metal-Aluminium Hydride Surrog[ATES]
Chem. Eur. J. 2022, e202201085
Banerjee S.;Macdonald P. A.; Orr S. A.; Kennedy A. R.; van Teijlingen A.; Robertson S. D.; Tuttle T.; Mulvey, R. E.
Chemistry - A European Journal 2022 e202201085
-
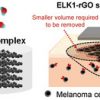
124. Disinfector-Assisted Low Temperature Reduced Graphene Oxide-Protein Surgical Dressing for the Postoperative Photothermal Treatment of Melanoma
Adv. Funct. Mater. 2022, 2205802.
Wu, Y.; Yang, J.; van Teijlingen, A.; Berardo, A.; Corridori, I.; Feng, J.; Xu, J.; Titirici, M.-M.; Rodriguez-Cabello, J. C.; Pugno, N. M.; Sun, J.; Wang, W.; Tuttle, T.; Mata, A.
Adv. Funct. Mater. 2022 2205802.
-
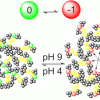
123. Constant pH Coarse-Grained Molecular Dynamics with Stochastic Charge Neutralization
J. Phys. Chem. Lett. 2022, 13, 4046.
van Teijlingen, A.; Swanson, H. W. A.; Lau, K. H. A.; Tuttle, T.
J. Phys. Chem. Lett. 2022 13 4046.
-

122. Directed discovery of tetrapeptide emulsifiers
Front. Chem. 2022, 10, 822868.
Scott, G. G.; Börner, T.; Leser, M. E.; Wooster, T. J.; Tuttle, T.
Front. Chem. 2022 10 822868.
-
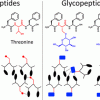
121. Expanding the Conformational Landscape of Minimalistic Tripeptides by Their O-Glycosylation
J. Am. Chem. Soc. 2021, 143, 19703.
Brito, A.; Dave, D.; Lampel, A.; Castro, V. I. B.; Kroiss, D.; Reis, R. L.; Tuttle, T.; Ulijn, R. V.; Pires, R. A.; Pashkuleva, I.
J. Am. Chem. Soc. 2021 143 19703
-
120. Radical and Ionic Mechanisms in Rearrangements of o-Tolyl Aryl Ethers and Amines Initiated by the Grubbs–Stoltz Reagent, Et3SiH/KOtBu
Molecules, 2021, 26, 6879.
Kolodziejczak, K.; Stewart, A. J.; Tuttle, T.; Murphy, j. A.
Molecules 2021 26 6879
-
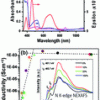
119. Understanding the dopant induced effects on SFX-MeOTAD for perovskite solar cells: a spectroscopic and computational investigation
J. Mater. Chem. C 2021, 9, 16226.
Gunn, F.; Ghosh, P.; Maciejczyk, M.; Cameron, J.; Dennis Nordlund, D.; Krishnamurthy, S.; Tuttle, T.; Skabara, P.; Robertson, N.; Ivaturi, A.
J. Mater. Chem. C. 2021 9 16226.
-
118. Reproduction of macroscopic properties of unsaturated triacylglycerides using a modified NERD force field
J. Mol. Graph. Model. 2021, 108, 107996.
Cordina, R. J.; Smith, B.; Tuttle, T.
J. Mol. Graph. Model. 2021 108 107996.
-
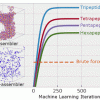
117. Beyond Tripeptides: Two-Step Active Machine Learning for Very Large Data sets
J. Chem. Theory Comput. 2021, 17, 3221.
van Teijlingen, A.; Tuttle, T.
J. Chem. Theory Comput. 2021 17 3221.
-
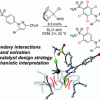
116. Catalyst design in C–H activation: a case study in the use of binding free energies to rationalise intramolecular directing group selectivity in iridium catalysis
Chem. Sci., 2021, 12, 6747.
Kerr, W. J.; Knox, G. J.; Reid, M.; Tuttle, T.
Chem. Sci. 2021 12 6747.
-

115. Mechanistic insights of evaporation-induced actuation in supramolecular crystals
Nature Mater. 2021, 20, 403.
Piotrowska, R.; Hesketh, T.; Wang, H.; Martin, A. R. G.; Bowering, D.; Zhang, C.; Hu, C. T.; McPhee, S. A.; Wang, T.; Park, Y.; Singla, P.; McGlone, T.; Florence, A.; Tuttle, T.; Ulijn, R. V.; Chen, X.
Nature Mater. 2021 20 403.
-
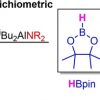
114. Structurally Defined Ring-Opening and Insertion of Pinacolborane into Aluminium-Nitrogen Bonds of Sterically Demanding Dialkylaluminium Amides.
Eur. J. Inor. Chem. 2021, 50.
Pollard, V. A.; Kennedy, A. R.; McLellan, R.; Ross, D.; Tuttle, T.; Mulvey, R. E.
Eur. J. Inorg. Chem. 2021 50.
-

113. Proton‐Conductive Melanin‐Like Fibers through Enzymatic Oxidation of a Self‐Assembling Peptide.
Adv. Mater. 2020, 32, 2003511.
Reddy, S. M. M.; Raßlenberg, E.; Sloan‐Dennison, S.; Hesketh, T.; Silberbush, O.; Tuttle, T.; Smith, E.; Graham, D.; Faulds, K.; Ulijn, R. V.; Ashkenasy, N.; Lampel, A.
Adv. Mater. 2020 32 2003511.
-
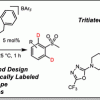
112. Computationally-Guided Development of a Chelated NHC-P Iridium(I) Complex for the Directed Hydrogen Isotope Exchange of Aryl Sulfones
ACS Catal. 2020, 10, 11120.
Kerr, W. J.; Knox, G. J.; Reid, M.; Tuttle, T.; Bergare, J.; Bragg, R. A.
ACS Catal 2020 10 11120.
-
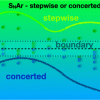
111. Computational Study on the Boundary Between the Concerted and Stepwise Mechanism of Bimolecular SNAr Reactions
J. Am. Chem. Soc. 2020, 142, 14871.
Rohrbach, S.; Murphy, J. A.; Tuttle, T.
J. Am. Chem. Soc. 2020 142 14871
-
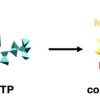
110. Combinatorial discovery and validation of heptapeptides with UTP‐binding induced structure
ChemSystemsChem 2020, 2, e2000025
Kroiss, D.; Aramini, J. M.; Ragoonath, S.; Ulijn, R. V.; Tuttle, T.
ChemSystemsChem 2020 2 e2000025
-
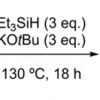
109. Benzylic C−H Functionalisation by [Et3SiH+KOtBu] leads to Radical Rearrangements in o‐ tolyl Aryl Ethers, Amines and Sulfides
Adv. Synth. Catal. 2020, 362, 2260.
Arokianathar, J. N.; Kolodziejczak, K.; Bugden, F. E.; Clark, K. F.; Tuttle, T.; Murphy, J. A.
Adv. Synth. Catal. 2020 362 2260.
-
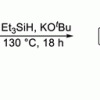
108. New reductive rearrangement of N-arylindoles triggered by the Grubbs–Stoltz reagent Et3SiH/KOtBu.
Chem. Sci. 2020, 11, 3719.
Smith, A. J.; Dimitrova, D.; Arokianathar, J. N.; Kolodziejczak, K.; Young, A.; Allison, M.; Poole, D. L.; Leach, S. G.; Parkinson, J. A.; Tuttle, T.; Murphy, J. A.
Chem. Sci. 2020 11 3719.
-
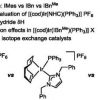
107. The Natural Product Lepidiline A as an N-Heterocyclic Carbene Ligand Precursor in Complexes of the Type [Ir(cod)(NHC)PPh3)]X: Synthesis, Characterisation, and Application in Hydrogen Isotope Exchange Catalysis
Catalysts, 2020, 10, 161.
Cochrane, A. R.; Kennedy, A. R.; Kerr, W. J.; Lindsay, D. M.; Reid, M.; Tuttle, T.
Catalysts 2020 10 161.
-
106. N‐Silylation of Amines Mediated by Et3SiH/KOtBu
HCA, 2019, 102, e1900235
Palumbo, F.; Rohrbach, S.; Tuttle, T.; Murphy, J. A.
HCA 2019 102 e1900235.
-

105. Concerted Nucleophilic Aromatic Substitution Reactions
Angew. Chem. Int. Ed. 2019, 58, 16368.
Rohrbach, S.; Smith, A. J.; Pang, J. H.; Poole, D. L.; Tuttle, T.; Chiba, S.; Murphy, J. A.
Angew. Chem. Int. Ed. 2019 58 16368.
-
104. Lithium‐Aluminate‐Catalyzed Hydrophosphination Applications
Agnew. Chem. Int. Ed. 2019, 58, 12291.
Pollard, V. A.; Young, A.; McLellan, R.; Kennedy, A. R.; Tuttle, T.; Mulvey, R. E.
Agnew. Chem. Int. Ed. 2019 58 12291.
-

103. Catalyst: Can Systems Chemistry Unravel the Mysteries of the Chemical Origins of Life?
CHEM, 2019, 5, 1917.
Kroiss, D.; Ashkenasy, G.; Braunschweig, A. B.; Tuttle, T.; Ulijn, R. V.
CHEM 2019 5 1917.
-
102. Neutral Organic Super Electron Donors Made Catalytic
Angew. Chem. Int. Ed. 2019, 58, 11454.
Rohrbach, S.; Shah, R. S.; Tuttle, T.; Murphy, J. A.
Angew. Chem. Int. Ed. 2019 58 11454.
-
101. Unbiased Discovery of Dynamic Peptide‐ATP Complexes
ChemSystemsChem, 2019, 1, 7.
Kroiss, D.; Aramini, J. M.; McPhee, S. A.; Tuttle, T.; Ulijn, R. V.
ChemSystemsChem 2019 1 7.
-
100. Computational prediction of tripeptide-dipeptide co-assembly
Mol. Phys. 2019, 117, 1151.
Moreira, I. P.; Scott, G. G.; Ulijn, R. V.; Tuttle, T.
Mol. Phys. 2019 117 1151
-
99. Dual Roles for Potassium Hydride in Haloarene Reduction: CSNAr and Single Electron Transfer Reduction via Organic Electron Donors Formed in Benzene
J. Am. Chem. Soc. 2018, 140, 11510.
Barham, J. P.; Dalton, S. E.; Allison, M.; Nocera, G.; Young, A.; John, M. P.; McGuire, T.; Campos, S.; Tuttle, T.; Murphy, J. A.
J. Am. Chem. Soc. 2018 140 11510
-
98. Electron Transfer Reactions: KOtBu (but not NaOtBu) Photoreduces Benzophenone under Activation by Visible Light
J. Am. Chem. Soc. 2018 140 9751.
Nocera, G.; Young, A; Palumbo, F.; Emery, K. J.; Coulthard, G.; McGuire, T.; Tuttle, T.; Murphy, J. A.
J. Am. Chem. Soc. 2018 140 9751.
-
97. Enhanced iridium complexes for elevated substrate applicability in isotope labelling processes.
J. Label Compd. Radiopharm. 2018, 61, 465.
Knox, G.; Kerr, W. J.; Tuttle, T.; Bragg, R.
J. Label Compd. Radiopharm. 2018 61 465
-
96. Guiding principles for peptide nanotechnology through directed discovery
Chem. Soc. Rev. 2018, 47, 3737.
Lampel, A.; Ulijn, R. V.; Tuttle, T.
Chem. Soc. Rev. 2018 47 3737.
-
95. KOtBu as a Single Electron Donor? Revisiting the Halogenation of Alkanes with CBr4 and CCl4
Molecules 2018, 23, 1055.
Emery, K. J.; Young, A.; Arokianathar, J. N.; Tuttle, T.; Murphy, J. A.
Molecules 2018 23 1055.
-
94. A Computational Study of Anionic Alkoxide–Allene and Amide–Allene Cyclizations
Eur. J. Org. Chem., 2017, 6867.
Cumine, F.; Young, A.; Reissig, H.-U.; Tuttle, T.; Murphy, J. A.
Eur. J. Org. Chem. 2017 6867.
-
93. Evidence of single electron transfer from the enolate anion of an N,N′-dialkyldiketopiperazine additive in BHAS coupling reactions
Org. Biomol. Chem., 2017, 15, 8810.
Emery, K. J.; Tuttle, T.; Murphy, J. A.
Org. Biomol. Chem. 2017 15 8810.
-
92. Electron-Transfer and Hydride-Transfer Pathways in the Stoltz–Grubbs Reducing System (KOtBu/Et3SiH)
Angew. Chem. Int. Ed. 2017 56 13747.
Smith, A. J.; Young, A.; Rohrbach, S.; O'Connor E. F.; Allison, M.; Wang, H-S.; Poole, D. L.; Tuttle, T.; Murphy, J. A.
Angew. Chem. Int. Ed. 2017 56 13747.
-
91. Site-Selective Deuteration of N-Heterocycles via Iridium-Catalyzed Hydrogen Isotope Exchange
ACS Catal., 2017, 7 (10), pp 7182–7186
Kerr, W. J.; Lindsay, D. M.; Owens, P. K.; Reid, M.; Tuttle, T.; Campos, S.
ACS Catal. 2017 7 7182.
-
90. New Insights into the Catalytic Mechanism of Aldose Reductase: A QM/MM Study
ACS Omega, 2017, 2 (9), pp 5737–5747
Dreanic, M. P.; Edge, C. M.; Tuttle, T.
ACS Omega 2017 2 5737.
-

89. Cooperative, ion-sensitive co-assembly of tripeptide hydrogels
Chem. Commun. 2017, 53, 9562.
Abul-Haija, Y. M.; Scott, G. G.; Sahoo, J. K.; Tuttle, T.; Ulijn, R. V.
Chem. Commun. 2017 53 9562.
-
88. Molecular dynamics simulations reveal disruptive self-assembly in dynamic peptide libraries
Org. Biomol. Chem., 2017, 15, 6541-6547
Sasselli, I. R.; Moreira, I. P.; Ulijn, R. V.; Tuttle, T.
Org. Biomol. Chem. 2017 15 6541.
-
87. Iridium-Catalyzed Formyl-Selective Deuteration of Aldehydes
Angew. Chem. Int. Ed. 2017 56 7808.
Kerr, W. J.; Reid, M.; Tuttle, T.
Angew. Chem. Int. Ed. 2017 56 7808.
-

86. Polymeric peptide pigments with sequence-encoded properties
Science 2017 356 1064.
Lampel, A.; McPhee, S. A.; Park, H-A.; Scott, G. G.; Humagain, S.; Hekstra, D. R.; Barney Yoo, B.; Frederix, P. W. J. M.; Li, T-D.; Abzalimov, R. R.; Greenbaum, S. G.; Tuttle, T.; Hu, C.; Betting, C. J.; Ulijn, R. V.
Science 2017 356 1064.
-
85. Biocatalytic Self-Assembly of Tripeptide Gels and Emulsions
Langmuir 2017 33 4986.
Moreira, I. P.; Piskorz, T. K.; van Esch, J. H.; Tell Tuttle, T.; Ulijn, R. V.
Langmuir 2017 33 4986.
-
84. A study of diketopiperazines as electron-donor initiators in transition metal-free haloarene–arene coupling.
Org. Biomol. Chem. 2017 15 3324.
Cumine, F.; Zhou, S.; Tuttle, T.; Murphy, J. A.
Org. Biomol. Chem. 2017 15 3324.
-
83. An Ambipolar BODIPY Derivative for a White Exciplex OLED and Cholesteric Liquid Crystal Laser toward Multifunctional Devices
ACS Appl. Mater. Interfaces 2017 9 4750.
Chapran, M.; Angioni, E.; Findlay, N. J.; Breig, B.; Vladyslav Cherpak, V.; Stakhira, P.; Tuttle, T.; Volyniuk, D.; Grazulevicius, J. V.; Nastishin, Y. A.; Lavrentovich, O. D.; Skabara, P. J.
ACS Appl. Mater. Interfaces 2017 9 4750.
-
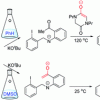
82. Effect of solvent on radical cyclisation pathways: SRN1 vs. aryl–aryl bond forming mechanisms
Org. Biomol. Chem. 2017 15 920.
Emery, K. J.; Murphy, J. A.; Tuttle, T.
Org. Biomol. Chem. 2017 15 920.
-
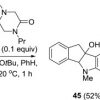
81. C–C bond-forming reactions of ground-state aryl halides under reductive activation
Tetrahedron 2016 72 7875.
Emery, K. J.; Tuttle, T.; Kennedy, A. R.; Murphy, J. A.
Tetrahedron 2016 72 7875.
-

80. Using experimental and computational energy equilibration to understand hierarchical self-assembly of Fmoc-dipeptide amphiphiles
Soft Matter 2016 12 8307.
Sasselli, I. R.; Pappas, C. G.; Matthews, E.; Wang, T.; Hunt, N. T.; Ulijn, R. V.; Tuttle, T.
Soft Matter 2016 12 8307.
-

79. Elucidation of the bonding of a near infrared dye to hollow gold nanospheres – a chalcogen tripod.
Chem. Sci. 2016 7 5160.
Kearns, H.; Sengupta, S.; Ramos Sasselli, I.; Bromley III, L.; Faulds, K.; Tuttle, T.; Bedics, M. A.; Detty, M. R.; Velarde, L.; Graham, D.; Smith, W. E.
Chem. Sci. 2016 7 5160.
-
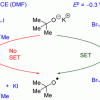
78. KOtBu: A Privileged Reagent for Electron Transfer Reactions?
J. Am. Chem. Soc. 2016 138 7402.
Barham, J. P.; Coulthard, G.; Emery, K. J.; Doni, E.; Cumine, F.; Nocera, G.; John, M. P.; Berlouis, L. E. A.; McGuire, T.; Tuttle, T.; Murphy, J. A.
J. Am. Chem. Soc. 2016 138 7402.
-
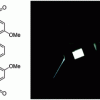
77. A single emitting layer white OLED based on exciplex interface emission
J. Mater. Chem. C 2016 4 3851.
Angioni, E.; Chapran, M.; Ivaniuk, K.; Kostiv, N.; Charpak, V.; Stakhira, P.; Lazauskas, A.; Tamulevičius, S.; Volyniuk, D.; Findlay, N. J.; Tuttle, T.; Grazulevicius, J. D.; Skabara, P. J.
J. Mater. Chem. C 2016 4 3851.
-
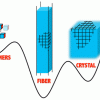
76. Supramolecular Fibers in Gels Can Be at Thermodynamic Equilibrium: A Simple Packing Model Reveals Preferential Fibril Formation versus Crystallization
ACS Nano 2016 10 2661.
Ivan Ramos Sasselli; Peter J. Halling; Rein V. Ulijn; Tell Tuttle
ACS Nano 2016 10 2661.
-
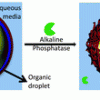
75. Enzymatically activated emulsions stabilised by interfacial nanofibre networks
Soft Matter 2016 12 2623.
Inês P. Moreira; Ivan Ramos Sasselli; Daniel A. Cannon; Meghan Hughes; Dimitrios A. Lamprou; Tell Tuttle; Rein V. Ulijn
Soft Matter 2016 12 2623.
-
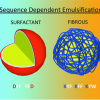
74. Tripeptide Emulsifiers
Adv. Materials 2016 28 1329
Gary G. Scott; Paul J. McKnight; Tell Tuttle; Rein V. Ulijn
Adv. Materials 2016 28 1329
-
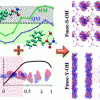
73. CHARMM force field parameterization protocol for self-assembling peptide amphiphiles: the Fmoc moiety
Phys. Chem. Chem. Phys. 2016 18 4659.
Ivan Ramos Sasselli; Rein V. Ulijn; Tell Tuttle
Phys. Chem. Chem. Phys. 2016 18 4659.
-
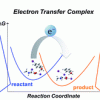
72. Predicting the reducing power of organic super electron donors
RSC Advances 2016 6 11335.
Greg M. Anderson; Iain Cameron; John A. Murphy; Tell Tuttle
RSC Advances 2016 6 11335.
-
71. Influence of Solvent in Controlling Peptide−Surface Interactions
J. Phys. Chem. Lett. 2015 6 3944.
Cannon, D. A.; Ashkenasy, N.; Tuttle, T.
J. Phys. Chem. Lett. 2015 6 3944.
-
70. Thiazole-induced rigidification in substituted dithieno-tetrathiafulvalene: the effect of planarisation on charge transport properties
Beilstein J. Org. Chem. 2015 11 1148
Taylor, R. G. D.; Cameron, J.; Wright, I. A.; Thomson, N.; Avramchenko, O.; Kanibolotsky, A. L.; Inigo, A. R.; Tuttle, T.; Skabara, P. J.
Beilstein J. Org. Chem. 2015 11 1148
-
69. Iridium-Catalysed ortho-Directed Deuterium Labelling of Aromatic Esters—An Experimental and Theoretical Study on Directing Group Chemoselectivity
Devlin, J.; Kerr, W. J.; Lindsay, D. M.; McCabe, T. J. D.; Reid, M.; Tuttle, T.
Molecules 2015 20 11676
-
68. Computational Approaches to Understanding the Self-assembly of Peptide-based Nanostructures
The interest in the self-assembly of peptide-based systems has grown significantly over the past 10–15 years, as more and more applications are shown to benefit from the useful properties of the amino acid based monomers. With the desire to apply the principals of self-assembly to systems within new application areas, there has been an increasing […]
Tuttle, T.
Isr. J. Chem. 2015 55 724
-

67. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels
Peptides that self-assemble into nanostructures are of tremendous interest for biological, medical, photonic and nanotechnological applications. The enormous sequence space that is available from 20 amino acids probably harbours many interesting candidates, but it is currently not possible to predict supramolecular behaviour from sequence alone. Here, we demonstrate computational tools to screen for the aqueous […]
Frederix, P. W. J. M.; Scott, G. G.; Abul-Haija, Y. M.; Kalafatovic, D.; Pappas, C. G.; Javid, N.; Hunt, N. T.; Ulijn, R. V.; Tuttle, T.
Nature Chem. 2015 7 30
-
66. Iridium-Catalyzed C-H Activation and Deuteration of Primary Sulfonamides: an Experimental and Computational Study
Iridium-catalyzed C-H activation and ortho-hydrogen isotope exchange is an important technology for allowing access to labelled organic substrates and aromatic drug molecules, and for the development of further C-H activation processes in organic synthesis. The use of [(COD)Ir(NHC)Cl] complexes (NHC = N-heterocyclic carbene) in the ortho-deuteration of primary sulfonamides under ambient conditions is reported. This […]
Kerr, W. J.; Reid, M.; Tuttle, T.
ACS Catal. 2015 5 402
-
65. Identifying the Roles of Amino Acids, Alcohols and 1,2-Diamines as Mediators in Coupling of Haloarenes to Arenes
Coupling of haloarenes to arenes has been facilitated by a diverse range of organic additives in the presence of KOtBu or NaOtBu since the first report in 2008. Very recently, we showed that the reactivity of some of these additives (e.g. compounds 6 and 7 in Figure 1) could be explained by the formation of […]
Shengze Zhou, S.-z.; Doni, E.; Anderson, G. M.; Kane, R. G.; MacDougall, S. W.; Ironmonger, V.; Tuttle, T.; Murphy, J. A.
J. Am. Chem. Soc. 2014 136 17818
-
64. Synthesis and properties of novel star-shaped oligofluorene conjugated systems with BODIPY cores
Beilstein J. Org. Chem. 2014 10 2704
Orofino-Pena, C.; Cortizo-Lacalle, D.; Cameron, J.; Sajjad, M. T.; Manousiadis, P. P.; Findlay, N. J.; Kanibolotsky, A. L.; Amarasinghe, D.; Skabara, P. J.; Tuttle, T.; Turnbull, G. A.; Samuel, I. D. W.
Beilstein J. Org. Chem. 2014 10 2704
-
63. Solution processable diketopyrrolopyrrole (DPP) cored small molecules with BODIPY end groups as novel donors for organic solar cells
Two novel triads based on a diketopyrrolopyrrole (DPP) central core and two 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) units attached by thiophene rings have been synthesised having high molar extinction coefficients. These triads were characterised and used as donor materials in small molecule, solution processable organic solar cells. Both triads were blended with PC71BM as an acceptor in different […]
Cortizo-Lacalle, D.; Howells, C. T.; Pandey, U. K.; Cameron, J.; Findlay, N. J.; Inigo, A. R.; Tuttle, T.; Skabara, P. J.; Samuel, I. D. W.
Beilstein J. Org. Chem. 2014 10 2683
-
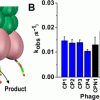
62. Discovery of Catalytic Phages by Biocatalytic Self-Assembly
Discovery of new catalysts for demanding aqueous reactions is challenging. Here, we describe methodology for selection of catalytic phages by taking advantage of localized assembly of the product of the catalytic reaction that is screened for. A phage display library covering 109 unique dodecapeptide sequences is incubated with nonassembling precursors. Phages which are able to […]
Maeda, Y.; Javid, N.; Duncan, K.; Birchall, L.; Gibson, K. F.; Cannon, D.; Kanetsuki, Y.; Knapp, C.; Tuttle, T.; Ulijn, R. V.; Matsui, H.
J. Am. Chem. Soc. 2014 136 15893
-
61. The Synthesis of Highly Active Iridium(I) Complexes and their Application in Catalytic Hydrogen Isotope Exchange
Brown, J. A.; Cochrane, A. R.; Irvine, S.; Kerr, W. J.; Mondal, B.; Parkinson, J. A.; Paterson, L. C.; Reid, M.; Tuttle, T.; Andersson, S.; Nilsson, G. N.
Adv. Synth. Catal. 2014 356 3551
-
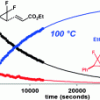
60. Evaluating the Thermal Vinylcyclopropane Rearrangement (VCPR) as a Practical Method for the Synthesis of Difluorinated Cyclopentenes: Experimental and Computational Studies of Rearrangement Stereospecificity
Orr, D.; Percy, J. M.; Tuttle, T.; Kennedy, A. R.; Harrison , Z. A.
Chem. Eur. J. 2014 20 14305
-
59. Investigation of the Ultrafast Dynamics Occurring during Unsensitized Photocatalytic H2 Evolution by an [FeFe]-Hydrogenase Subsite Analogue
Frederix, P. W. J. M.; Adamczyk, K.; Wright, J. A.; Tuttle, T.; Ulijn, R. V.; Pickett, C. J.; Hunt, N. T.
Organometallics 2014 33 5888
-
58. Forming a ruthenium isomerisation catalyst from Grubbs II: a DFT study
Young, A.; Vincent, M. A.; Hillier, I. H.; Percy, J. M.; Tuttle, T.
Dalton Trans. 2014 43 8493
-
57. Practically convenient and industrially-aligned methods for iridium-catalysed hydrogen isotope exchange processes
Cochrane, A. R.; Idziak, C.; Kerr, W. J.; Mondal, B.; Paterson, L. C.;Tuttle, T.; Andersson, S.;Nilsson, G. N.
Org. Biomol. Chem. 2014 12 3598
-
56. Metal-Free Reductive Cleavage of C—N and S—N Bonds by Photoactivated Electron Transfer from a Neutral Organic Donor
O’Sullivan, S.; Doni, E.; Tuttle, T.; Murphy, J. A.
Angew. Chem. Int. Ed. 2014 53 474
-
55. Organic Super-Electron-Donors: Initiators in Transition Metal-Free Haloarene-Arene Coupling.
Zhou, S.; Anderson, G. M.; Mondal, B.; Doni, E.; Ironmonger, V.; Kranz, M.; Tuttle, T.; Murphy, J. A.
Chem. Sci. 2014 5 476
-
54. Towards a Quantitative Understanding of Palladium Metal Scavenger Performance: An Electronic Calculation Structure Approach
Mondal, B.; Wilkes, R. D.; Percy, J. M.; Tuttle, T.; Black, R. J. G.; North, C.
Dalton Trans. 2014 43 469
-
53. Aromatic Peptide Amphiphiles: Significance of the Fmoc Moiety
Fleming, S.; Debnath, S.; Frederix, P. W. J. M.; Tuttle, T.; Ulijn, R. V.
Chem. Commun. 2013 49 10587
-
52. Overturning Established Chemoselectivities: Selective Reduction of Arenes over Malonates and Cyanoacetates by Photoactivated Organic Electron Donors
Doni, E.; Mondal, B.; O’Sullivan, S.; Tuttle, T.; Murphy, J. A.
J. Am. Chem. Soc. 2013 135 10934
-
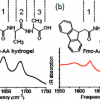
51. Assessing the Utility of Infrared Spectroscopy as a Structural Diagnostic Tool for β-Sheets in Self-Assembling Aromatic Peptide Amphiphiles
Fleming, S.; Frederix, P. W. J. M.; Ramos-Sasselli, I.; Hunt, N. T.; Ulijn, R. V.; Tuttle, T.
Langmuir 2013 29 9510
-
50. Incorporation of perfluorohexyl-functionalised thiophenes into oligofluorene-truxenes: synthesis and physical properties.
Thomson, N.; Kanibolotsky, A. L.; Cameron, J.; Tuttle, T.; Findlay, N. J.; Skabara, P. J.
Beilstein J. Org. Chem. 2013 9 1243
-
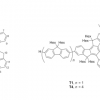
49. Organobase-Catalyzed Amidation of Esters with Amino Alcohols
Caldwell, N.; Jamieson, C.; Simpson, I.; Tuttle, T.
Org. Lett. 2013 15 2506
-
48. Stabilization of metastable hydrogen trioxide (HOOOH) and the hydrotrioxyl radical (HOOO•) by complexation with sulfuric acid. A theoretical study.
Cannon, D.; Tuttle, T.; Koller, J.; Plesnicar, B.
Comp. Theo. Chem. 2013 1010 19
-
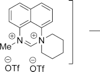
47. Superelectrophilic Amidine Dications: Dealkylation by Triflate Anion
Kovacevic, L. S.; Idziak, C.; Markevicius, A.; Scullion, C.; Corr, M. J.; Kennedy, A. R.; Tuttle, T.; Murphy, J. A.
Angew. Chem. Int. Ed. 2012 51 8156
-
46. Sequence/structure relationships in aromatic dipeptide hydrogels formed under thermodynamic control by enzyme-assisted self-assembly
Hughes, M.; Frederix, P. W. J. M.; Raeburn, J.; Birchall, L. S.; Sadownik, J.; Coomer, F. C.; Lin, I-H.; Cussen, E. J.; Hunt, N. T.; Tuttle, T.; Webb, S. J.; Adams, D. J.; Ulijn, R. V.
Soft Matter. 2012 8 5595
-
45. Oligothiophene Cruciform with a Germanium Spiro Center: A Promising Material for Organic Photovoltaics
Wright, I. A.; Kanibolotsky, A. L.; Cameron, J.; Tuttle, T.; Skabara, P. J.; Coles, S. J.; Howells, C. T.; Thomson, S. A. J.; Gambino, S.; Samuel, I. D. W.
Angew. Chem. Int. Ed. 2012 51 4562
-
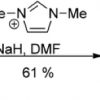
44. Imidazole-derived carbenes and their elusive tetraazafulvalene dimers
Jolly, P. I.; Zhou, S.; Thomson, D. W.; Garnier, J.; Parkinson, J. A.; Tuttle, T.; Murphy, J. A.
Chem. Sci. 2012 3 1675
-

43. Quantum Mechanical/Molecular Mechanical Approaches in Drug Design
Tuttle, T.
Drug Design Strategies Computational Techniques and Applications Eds. Banting, L.; Clark, T. 1
-
42. Tungsten(VI) N-Heterocyclic Carbene Complexes: Synthetic, Structural and Computational Study
Dodds, C. A.; Spicer, M. D.; Tuttle, T.
Organometallics 2011 30 6262