Publications
-
40. Virtual Screening for Dipeptide Aggregation: Toward Predictive Tools for Peptide Self-Assembly
Frederix, P. W. J. M.; Ulijn, R. V.; Hunt, N. T.; Tuttle, T.
J. Phys. Chem. Lett. 2011 2 2380
-
39. Main Group Multiple C-H/N-H Bond Activation of a Diamine and Isolation of a Molecular Dilithium Zincate Hydride: Experimental and DFT Evidence for Alkali Metal-Zinc Synergistic Effects.
Campbell, R.; Cannon, D.; Garcia-Alvarez, P.; Kennedy, A. R.; Mulvey, R. E.; Robertson, S. D.; Sassmannshausen, J.; Tuttle, T.
J. Am. Chem. Soc. 2011 133 13706
-
38. Exploiting the CH-π Interactions in Supramolecular Hydrogels of Aromatic Carbohydrate Amphiphiles
Birchall, L. S.; Roy, S.; Jayawarna, V.; Hughes, M.; Irvine, E.; Okorogheye, G. T.; Saudi, N.; De Santis, E.; Tuttle, T.; Edwards, A. A.; Ulijn, R. V.
Chem. Sci. 2011 2 1349
-
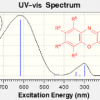
37. Predicting the UV-Vis Spectra of Oxazine Dyes
Fleming, S.; Mills, A.; Tuttle, T.
Beilstein J. Org. Chem. 2011 7 432
-
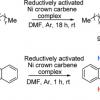
36. Reductions of Challenging Organic Substrates by a Nickel Complex of a Noninnocent Crown Carbene Ligand
Findlay, N. J.; Park, S. R.; Schoenebeck, F.; Cahard, E.; Zhou, S-z.; Berlouis, L. E. A.; Spicer, M. D.; Tuttle, T.; Murphy, J. A.
J. Am. Chem. Soc. 2010 132 15462
-
35. Fmoc hydrogels from aromatic carbohydrate amphiphiles
Edwards, A. A.; Birchall, L. S.; Jayawarna, V.; Roy, S.; Hughes, M.; Tuttle, T.; Saudi, N.; Okorgheye, G.; Ulijn, R. V.
J. Pharm. Pharmac. 2010 62 1331
-
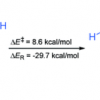
34. The Search for Protonated Dihydrogen Trioxide (HOOOH): Insights from Theory and Experiment
Tuttle, T.; Cerkovnik, J.; Koller, J.; Plesnicar, B.
J. Phys. Chem. A 2010 114 8003
-

33. Applications of QM/MM in Inorganic Chemistry.
Tuttle, T.
Spectrosc. Prop. Inorg. Organomet. Compd. 2010 41 87
-
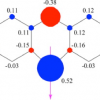
32. Effect of Alkali on Methylene Blue (C.I. Basic Blue 9) and other Thiazine Dyes.
Mills, A.; Hazafy, D.; Parkinson, J. A.; Tuttle, T.; Hutchings, M. G.
Dyes and Pigments 2010 88 149
-
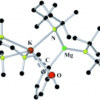
31. Structural Tracking of the Potassium-Mediated Magnesiation of Anisole.
Clegg, W.; Conway, B.; Garcia-Alvarez, P.; Kennedy, A. R.; Mulvey, R. E.; Russo, L.; Sassmannshausen, J.; Tuttle, T.
Chem. Eur. J. 2009 15 10702
-
30. Comment on “Solvent Effect on the Electronic Spectra of Azine Dyes under Alkaline Condition”
Mills, A.; Hazafy, D.; Parkinson, J. A.; Tuttle, T.; Hutchings, M. G.
J. Phys. Chem. A 2009 113 9575
-

29. Averaging Semiempirical NMR Chemical Shifts: Dynamic Effects on the Subpicosecond Time Scale.
Tuttle, T.
J. Phys. Chem. A 2009 113 11723
-
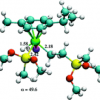
28. Ruthenium based catalysts for olefin hydrosilylation: dichloro(p-cymene)ruthenium and related complexes
Tuttle, T.; Wang, D.; Thiel, W.; Kohler, J.; Hofmann, M.; Weis, J.
Dalton Trans. 2009 5894
-
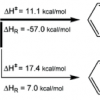
27. Hydrotrioxides Rather than Cyclic Tetraoxides (Tetraoxolanes) as the Primary Reaction Intermediates in the Low Temperature Ozonation of Aldehydes. The Case of Benzaldehyde
Cerkovnik, J.; Plesnicar, B.; Koller, J.; Tuttle, T.
J. Org. Chem. 2009 74 96
-
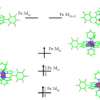
26. The Electronic Structure of Iron Corroles: A Combined Experimental and Quantum Chemical Study.
Ye, S.; Tuttle, T.; Bill, E.; Simkhovich, L.; Gross, Z.; Thiel, W.; Neese, F.
Chem. Eur. J. 2008 14 10839
-
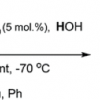
25. An Efficient Methyltrioxorhenium(VII)-Catalyzed Transformation of Hydrotrioxides (ROOOH) into Dihydrogen Trioxide (HOOOH).
Bergant, A.; Cerkovnik, J.; Plesnicar, B.; Tuttle, T.
J. Am. Chem. Soc. 2008 130 14086
-
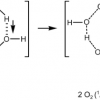
24. Dihydrogen Trioxide Clusters, (HOOOH)n (n = 2-4), and the Hydrogen-bonded Complexes of HOOOH with Acetone and Dimethyl Ether: Implications for the Decomposition of HOOOH
Kovacic, S.; Koller, J.; Cerkovnik, J.; Tuttle, T.; Plesnicar, B.
J. Phys. Chem. A 2008 112 8129
-
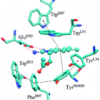
23. OMx-D: Semiempirical Methods with Orthogonalization and Dispersion Corrections. Implementation and Biochemical Application
Tuttle, T.; Thiel, W.
Phys. Chem. Chem. Phys. 2008 10 2159
-
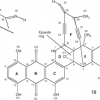
22. Design of a New Warhead for the Natural Enediyne Dynemicin A. An Increase of Biological Activity.
Kraka, E.; Tuttle, T.; Cremer, D.
J. Phys. Chem. B 2008 112 2661
-
21. The Reductive Cleavage of Sulfones and Sulfonamides By a Neutral Organic Super-Electron Donor (s.e.d) Reagant.
Schöenebeck, F.; Murphy, J. A.; Zhou, S-z.; Uenoyama, Y.; Miclo, Y.; Tuttle, T.
J. Am. Chem. Soc. 2007 129 13368
-

20. The Reactivity of Calicheamicin Gamma1I in the Minor Groove of DNA: The Decisive Role of the Environment
Kraka, E.; Tuttle, T.; Cremer, D.
Chem. Eur. J. 2007 13 9256
-
19. A QM/MM Study of the Bergman Reaction of Dynemicin A in the Minor Groove of DNA
Tuttle, T.; Kraka, E.; Thiel, W.; Cremer, D.
J. Phys. Chem. B, 2007 111 8321
-
18. Substrate Orientation in 4-oxalocrotonate Tautomerase and its Effect on QM/MM Energy Profiles.
Tuttle, T.; Thiel, W.
J. Phys. Chem. B 2007 111 7665
-
17. The Generation of Aryl Anions By Double Electron Transfer To Aryl Iodides From a Neutral Ground-state Organic Super-electron Donor.
Murphy, J. A.; Zhou, S-z.; Thomson, D. W.; Schöenebeck, F.; Mahesh, M.; Park, S. R.; Tuttle, T.; Berlouis, L. E. A.
Angew. Chem. Int. Ed. 2007 46 5178
-
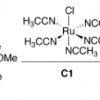
16. Mechanism of Olefin Hydrosilylation Catalyzed By [RuCl(NCCH3)5]+: a DFT Study.
Tuttle, T.; Wang, D.; Thiel, W.; Köhler, J.; Hofmann, M.; Weis, J.
J. Organomet. Chem. 2007 692 2282
-
15. Latrunculin Analogues with Improved Biological Profiles By “diverted Total Synthesis”: Preparation, Evaluation and Computational Analysis.
Fürstner, A.; Kirk, D.; Fenster, M. D. B.; Aïssa, C.; De Souza, D.; Nevado, C.; Tuttle, T.; Thiel, W.; Müller, O.
Chem. Eur. J. 2007 13 135
-
14. Understanding the Enzymatic Activity of 4-oxalocrotonate and its Mutant Analogs: a Computational Study.
Tuttle, T.; Keinan, E.; Thiel, W.
J. Phys. Chem. B 2006 110 19685
-
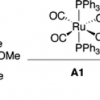
13. Mechanism of Olefin Hydrosilylation Catalyzed By RuCl2(CO)2(PPh3)2: a DFT Study.
Tuttle, T.; Wang, D.; Thiel, W.; Köhler, J.; Hofmann, M.; Weis, J.
Organometallics 2006 25 4504
-
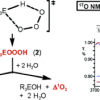
12. The Ozonation of Silanes and Germanes: An Experimental and Theoretical Investigation
Cerkovnik, J.; Tuttle, T.; Kraka, E.; Lendero, N.; Plesnicar, B.; Cremer, D.
J. Am. Chem. Soc. 2006 128 4090
-

11. Docking, Triggering, and Biological Activity of Dynemicin a in DNA: a Computational Study.
Tuttle, T.; Kraka, E.; Cremer, D.
J. Am. Chem. Soc. 2005 127 9469
-
10. Elucidation of the Electronic Structure of Molecules with the Help of NMR Spin-spin Coupling Constants: the FH Molecule.
Grafenstein, J.; Tuttle, T.; Cremer, D.
J. Phys. Chem. A 2005 109 2325
-
9. Analysis of Long-range NMR Spin-spin Coupling in Polyenes and the π-mechanism.
Grafenstein, J.; Tuttle, T.; Cremer, D.
Phys. Chem. Chem. Phys. 2005 7 452
-

8. Hemiortho Esters and Hydrotrioxides As the Primary Products in the Low-temperature Ozonation of Cyclic Acetals: An Experimental and Theoretical Investigation.
Tuttle, T.; Cerkovnik, J.; Plesnicar, B.; Cremer, D.
J. Am. Chem. Soc. 2004 126 16093
-
7. Analysis of the NMR Through-space Coupling Mechanism Between F-19 Atoms.
Tuttle, T.; Grafenstein, J.; Cremer, D.
Chem. Phys. Lett. 2004 394 5
-

6. Decomposition of Nuclear Magnetic Resonance Spin-spin Coupling Constants Into Active and Passive Orbital Contributions.
Grafenstein, J.; Tuttle, T.; Cremer, D.
J. Chem. Phys. 2004 120 9952
-
5. Investigation of the Nmr Spin-spin Coupling Constants Across the Hydrogen Bonds in Ubiquitin: the Nature of the Hydrogen Bond As Reflected By the Coupling Mechanism.
Tuttle, T.; Kraka, E.; Wu, A.; Cremer, D.
J. Am. Chem. Soc. 2004 126 5093
-
4. Analysis of the NMR Spin−Spin Coupling Mechanism Across a H−Bond: Nature of the H-Bond in Proteins
Tuttle, T.; Grafenstein, J.; Wu, A.; Kraka, E.; Cremer, D.
J. Phys. Chem. B 2004 108 1115
-
3. Molecular Modelling and Design of Radiolabelled Complexes for Melanoma Diagnosis.
Tuttle, C.T.T.; Seabrook, S.A.; Wise, L.E.; Knott, R.B.; Yates, B.F.
Aust. J. Chem. 2004 57 87
-
2. Mechanism of Formation of Hydrogen Trioxide (HOOOH) in the Ozonation of 1,2-Diphenylhydrazine and 1,2-Dimethylhydrazine: An Experimental and Theoretical Investigation
Low-temperature (−78 °C) ozonation of 1,2-diphenylhydrazine in various oxygen bases as solvents (acetone-d6, methyl acetate, tert-butyl methyl ether) produced hydrogen trioxide (HOOOH), 1,2-diphenyldiazene, 1,2-diphenyldiazene-N-oxide, and hydrogen peroxide ...
Plesnicar, B.; Tuttle, T.; Cerkovnik, J.; Koller, J.; Cremer, D.
J. Am. Chem. Soc. 2003 125 11553
-
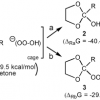
1. Evidence for the HOOO- Anion in the Ozonation of 1,3-Dioxolanes: Hemiortho Esters as the Primary Product
Low-temperature ozonation (−78 °C) of 2-methyl-1,3-dioxolane (1a) in acetone-d6, methyl acetate, and tert-butyl methyl ether produced both the corresponding acetal hydrotrioxide (3a, ROOOH) and the hemiortho ester (2a, ROH) in molar ratio 1:5. Both intermediates were fully characterized by 1H, 13C, and 17O NMR spectroscopy, and they both decomposed to the corresponding hydroxy ester at higher temperatures. The […]
Plesnicar, B.; Cerkovnik, J.; Tuttle, T.; Kraka, E.; Cremer, D.
J. Am. Chem. Soc. 2002 124 11260.