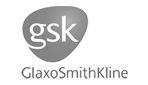Reactivity
-

141. Iridium-Catalysed C(sp3)−H Activation and Hydrogen Isotope Exchange via Nitrogen-Based Carbonyl Directing Groups
Adv. Synth. Catal. 2024 doi.org/10.1002/adsc.202400156
Knight, N. M. L,; Thompson, J. D. F.; Parkinson, J. A.; Lindsay, D. M.; Tuttle, T.; Kerr, W. J.
Adv. Synth. Catal. 2024 doi.org/10.1002/adsc.202400156
-
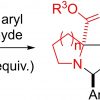
135. [3+ 2]‐Cycloaddition Reactions of gem‐Difluorocyclopropenes with Azomethine Ylides–Access to Novel Fluorinated Scaffolds
Chem. Eur. J. 2023, e202301861, 10.1002/chem.202301861
Donnelly, K.; Singh, A.; Tuttle, T.; Baumann, M.
Chem. Eur. J. 2023 e202301861
-
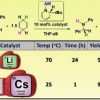
134. Alkali Metal Dihydropyridines in Transfer Hydrogenation Catalysis of Imines: Amide Basicity versus Hydride Surrogacy
Angew. Chem. Int. Ed., 2023, e202304966, 10.1002/anie.202304966
Macdonald, P. A.; Banerjee, S,; Kennedy, A. R.; van Teijlingen, A.; Robertson, S. D.; Tuttle, T.; Mulvey, R. E.
Angew. Chem. Int. Ed. 2023 e202304966
-
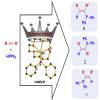
126. Catalytic hydrophosphination of alkynes using structurally diverse sodium diphenylphosphide donor complexes
Cell Reports Physical Science., 2022, 100942
Whitelaw, M.; Banerjee, S.; Kennedy, A.; van Teijlingen, A.; Tuttle, T.; Mulvey, R.
Cell Reports Physical Chemistry 2022 100942