2. Mechanism of Formation of Hydrogen Trioxide (HOOOH) in the Ozonation of 1,2-Diphenylhydrazine and 1,2-Dimethylhydrazine: An Experimental and Theoretical Investigation
Low-temperature (−78 °C) ozonation of 1,2-diphenylhydrazine in various oxygen bases as solvents (acetone-d6, methyl acetate, tert-butyl methyl ether) produced hydrogen trioxide (HOOOH), 1,2-diphenyldiazene, 1,2-diphenyldiazene-N-oxide, and hydrogen peroxide ...
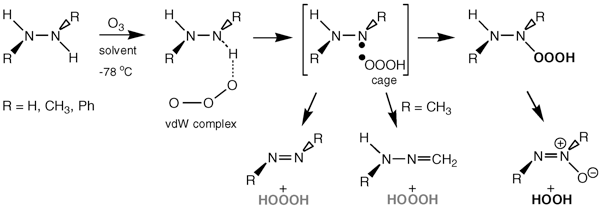
Low-temperature (−78 °C) ozonation of 1,2-diphenylhydrazine in various oxygen bases as solvents (acetone-d6, methyl acetate, tert-butyl methyl ether) produced hydrogen trioxide (HOOOH), 1,2-diphenyldiazene, 1,2-diphenyldiazene-N-oxide, and hydrogen peroxide. Ozonation of 1,2-dimethylhydrazine produced besides HOOOH, 1,2-dimethyldiazene, 1,2-dimethyldiazene-N-oxide and hydrogen peroxide, also formic acid and nitromethane. Kinetic and activation parameters for the decomposition of the HOOOH produced in this way, and identified by 1H, 2H, and 17O NMR spectroscopy, are in agreement with our previous proposal that water participates in this reaction as a bifunctional catalyst in a polar decomposition process to produce water and singlet oxygen (O2, 1Δg). The possibility that hydrogen peroxide is, besides water, also involved in the decomposition of hydrogen trioxide is also considered. The half-life of HOOOH at room temperature (20 °C) is 16 ± 1 min in all solvents investigated. Using a variety of DFT methods (restricted, broken-symmetry unrestricted, self-interaction corrected) in connection with the B3LYP functional, a stepwise mechanism involving the hydrotrioxyl (HOOO•) radical is proposed for the ozonation of hydrazines (RNHNHR, R = H, Ph, Me) that involves the abstraction of the N-hydrogen atom by ozone to form a radical pair, RNNHR• •OOOH. The hydrotrioxyl radical can then either abstract the remaining N(H) hydrogen atom from the RNNHR•radical to form the corresponding diazene (RN=NR), or recombines with RNNHR• in a solvent cage to form the hydrotrioxide, RN(OOOH)NHR. The decomposition of these very labile hydrotrioxides involves the homolytic scission of the RO−OOH bond with subsequent “in cage” formation of the diazene-N-oxide and hydrogen peroxide. Although 1,2-diphenyldiazene is unreactive toward ozone under conditions investigated, 1,2-dimethyldiazene reacts with relative ease to yield 1,2-dimethyldiazene-N-oxide and singlet oxygen (O2, 1Δg). The subsequent reaction sequence between these two components to yield nitromethane as the final product is discussed. The formation of formic acid and nitromethane in the ozonolysis of 1,2-dimethylhydrazine is explained as being due to the abstraction of a methyl H atom of the CH3NNHCH3• radical by HOOO• in the solvent cage. The possible mechanism of the reaction of the initially formed formaldehyde methylhydrazone (and HOOOH) with ozone/oxygen mixtures to produce formic acid and nitromethane is also discussed.