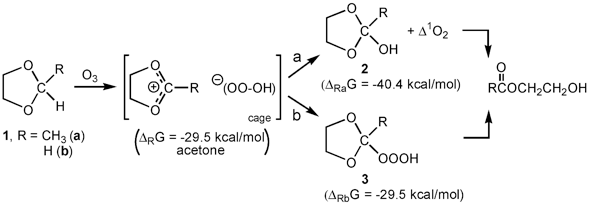
1. Evidence for the HOOO- Anion in the Ozonation of 1,3-Dioxolanes: Hemiortho Esters as the Primary Product
Low-temperature ozonation (−78 °C) of 2-methyl-1,3-dioxolane (1a) in acetone-d6, methyl acetate, and tert-butyl methyl ether produced both the corresponding acetal hydrotrioxide (3a, ROOOH) and the hemiortho ester (2a, ROH) in molar ratio 1:5. Both intermediates were fully characterized by 1H, 13C, and 17O NMR spectroscopy, and they both decomposed to the corresponding hydroxy ester at higher temperatures. The mechanism involving the HOOO–anion formed by the abstraction of the hydride ion by ozone to form an ion pair, R+ –OOOH, with its subsequent collapse to either the corresponding hemiortho ester (ROH) or the acetal hydrotrioxide (ROOOH) was proposed. This mechanism is supported by the PISA/B3LYP/6-311++G(3df,3pd) calculations.