Publications
-
1. Evidence for the HOOO- Anion in the Ozonation of 1,3-Dioxolanes: Hemiortho Esters as the Primary Product
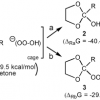
Low-temperature ozonation (−78 °C) of 2-methyl-1,3-dioxolane (1a) in acetone-d6, methyl acetate, and tert-butyl methyl ether produced both the corresponding acetal hydrotrioxide (3a, ROOOH) and the hemiortho ester (2a, ROH) in molar ratio 1:5. Both intermediates were fully characterized by 1H, 13C, and 17O NMR spectroscopy, and they both decomposed to the corresponding hydroxy ester at higher temperatures. The […]
Plesnicar, B.; Cerkovnik, J.; Tuttle, T.; Kraka, E.; Cremer, D.
J. Am. Chem. Soc. 2002 124 11260.