Publications
-
64. Synthesis and properties of novel star-shaped oligofluorene conjugated systems with BODIPY cores
Beilstein J. Org. Chem. 2014 10 2704
Orofino-Pena, C.; Cortizo-Lacalle, D.; Cameron, J.; Sajjad, M. T.; Manousiadis, P. P.; Findlay, N. J.; Kanibolotsky, A. L.; Amarasinghe, D.; Skabara, P. J.; Tuttle, T.; Turnbull, G. A.; Samuel, I. D. W.
Beilstein J. Org. Chem. 2014 10 2704
-
63. Solution processable diketopyrrolopyrrole (DPP) cored small molecules with BODIPY end groups as novel donors for organic solar cells
Two novel triads based on a diketopyrrolopyrrole (DPP) central core and two 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) units attached by thiophene rings have been synthesised having high molar extinction coefficients. These triads were characterised and used as donor materials in small molecule, solution processable organic solar cells. Both triads were blended with PC71BM as an acceptor in different […]
Cortizo-Lacalle, D.; Howells, C. T.; Pandey, U. K.; Cameron, J.; Findlay, N. J.; Inigo, A. R.; Tuttle, T.; Skabara, P. J.; Samuel, I. D. W.
Beilstein J. Org. Chem. 2014 10 2683
-
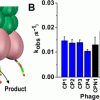
62. Discovery of Catalytic Phages by Biocatalytic Self-Assembly
Discovery of new catalysts for demanding aqueous reactions is challenging. Here, we describe methodology for selection of catalytic phages by taking advantage of localized assembly of the product of the catalytic reaction that is screened for. A phage display library covering 109 unique dodecapeptide sequences is incubated with nonassembling precursors. Phages which are able to […]
Maeda, Y.; Javid, N.; Duncan, K.; Birchall, L.; Gibson, K. F.; Cannon, D.; Kanetsuki, Y.; Knapp, C.; Tuttle, T.; Ulijn, R. V.; Matsui, H.
J. Am. Chem. Soc. 2014 136 15893
-
61. The Synthesis of Highly Active Iridium(I) Complexes and their Application in Catalytic Hydrogen Isotope Exchange
Brown, J. A.; Cochrane, A. R.; Irvine, S.; Kerr, W. J.; Mondal, B.; Parkinson, J. A.; Paterson, L. C.; Reid, M.; Tuttle, T.; Andersson, S.; Nilsson, G. N.
Adv. Synth. Catal. 2014 356 3551