66. Iridium-Catalyzed C-H Activation and Deuteration of Primary Sulfonamides: an Experimental and Computational Study
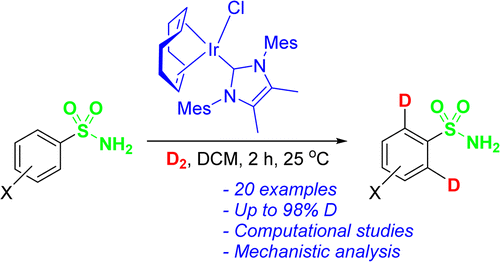
Iridium-catalyzed C-H activation and ortho-hydrogen isotope exchange is an important technology for allowing access to labelled organic substrates and aromatic drug molecules, and for the development of further C-H activation processes in organic synthesis. The use of [(COD)Ir(NHC)Cl] complexes (NHC = N-heterocyclic carbene) in the ortho-deuteration of primary sulfonamides under ambient conditions is reported. This methodology has been applied to the deuteration of a series of substrates, including the COX-2 inhibitors Celecoxib and Mavacoxib, demonstrating selective complexation of the primary sulfonamide over a competing pyrazole moiety. The observed chemoselectivity can be reversed by employing more encumbered catalyst derivatives of the type [(COD)Ir(NHC)(PPh3)]PF6. Computational studies have revealed that, although C-H activation is rate-determining, substrate complexation or subsequent C-H activation can be product-determining depending on the catalyst employed.